Social
Product Safety and Quality
Product Safety Assurance Policy
Legal compliance
Each company in the HOYA Group complies with the standards required of products in Japan and elsewhere around the world. We also comply with laws, regulations, and other standards related to product quality and safety.
Collecting and responding to product incident information
Each company in the HOYA Group collects information from customers and other sources related to product incidents and/or malfunctions. When necessary, we provide legally mandated reports to regulatory agencies and appropriate information to customers and others. We enact measures to recall products, repair products, in addition to taking other actions regarding any product-related issues.
Ensuring product safety
Each company in the HOYA Group provides, where appropriate, user training, up-to-date user guides and documents, product warning labels, and other information for the effective, safe use of our products. We continue to seek opinions from users of our products and reflect this feedback in future products.
Training and educational systems to improve product safety and quality
Each company in the HOYA Group strives to improve product safety and quality. To accomplish this goal, we conduct ongoing education and training activities. At the same time, each Group company performs reviews and updates to product safety and quality management organizations and systems.
Product Safety Assurance Structure
At the HOYA Group, under the supervision of the executive officer in charge, each business division has product safety assurance functions. For businesses falling under the Life Care segment, which handles healthcare and medical products, we have established a division at the head office in charge of regulations, quality, and government affairs across these businesses.
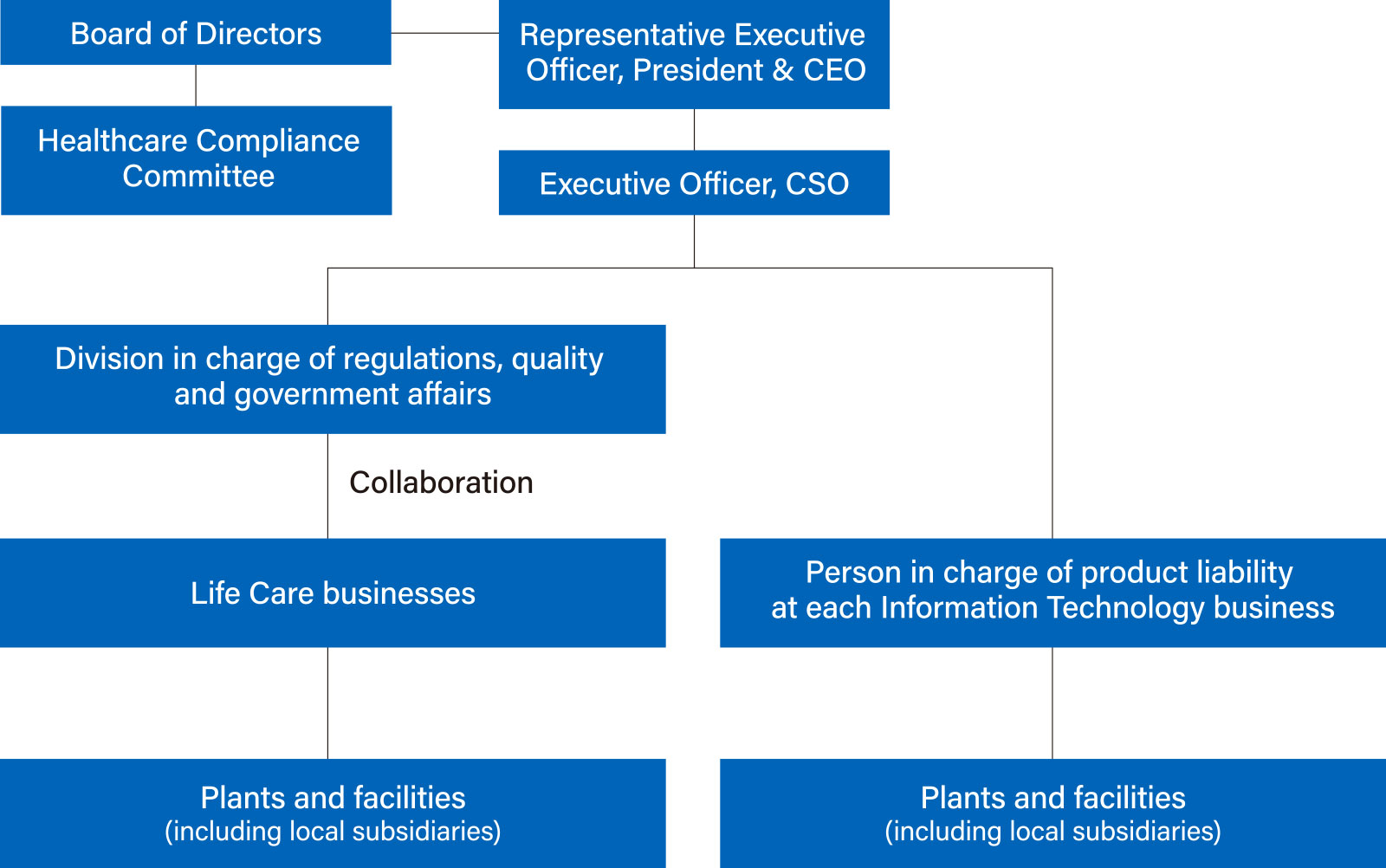
Healthcare Compliance Committee
The HOYA Group established the Healthcare Compliance Committee with the objective of obtaining the thorough compliance with laws, ordinances, standards and norms, both in Japan and overseas, required of healthcare products. Composed of three independent directors who are knowledgeable about the healthcare field, the Healthcare Compliance Committee conducts monitoring of the status of the relevant business divisions’ responses to regulations and other matters. Obtaining advice as necessary from specialists with expert knowledge of the laws and ordinances in each country with respect to healthcare products, each member of the Healthcare Compliance Committee offers proposals and advice to each business division for whose regulatory response he or she is responsible. In addition, the chairperson of the Healthcare Compliance Committee reports the details of the deliberations of each meeting of the Healthcare Compliance Committee to the Board of Directors.
Quality Management System
The HOYA Group considers product safety and quality to be fundamental to its corporate value and has acquired international quality management system ISO9001 (mainly for the Information Technology business) or ISO13485 (mainly for the Life Care business) focusing on major production bases in each business.
This enables the Group to strengthen its quality assurance system in product design, manufacturing, and sales, thereby improving reliability in the global market.
With regard to product safety, we comply with domestic and international laws and regulations and industry standards, and through the collection and analysis of product accident and defect information, we promptly take necessary measures such as reporting to regulatory authorities and conducting product recalls or modifications. Also, a structure has been established to incorporate user feedback and reflect it in improvements to future products.
Internally, we conduct ongoing education and training on product safety and quality control to raise employee awareness and skills and are reviewing and improving the quality control organizational structure in each business division. For businesses falling under the Life Care segment, which handles medical products, we have established a division at the head office in charge of regulations, quality, and government affairs across these businesses to comprehensively manage product safety and legal compliance.
Going forward, the HOYA Group will continue to strive to further improve product safety and quality, strengthen its management system based on international standards, and enhance customer satisfaction.
Status of ISO certification
ISO9001 |
ISO14001 |
ISO45001 |
ISO13485 |
|
|---|---|---|---|---|
Number of ISO-certified sites*1 |
35(1) |
50(3) |
48(4) |
53(0) |
Uptake rate*2 |
100% |
96% |
92% |
– |
*1 The number in parenthesis is the change from the previous year.
*2 Refers to the percentage of production sites eligible for certification that have completed certification.
For information on our acquisition status of ISO certification, please refer to this page
Animal Experimentation
There are some cases in which animal experiments need to be conducted to develop some medical products. HOYA Group has established regulations in each business division in consideration of various laws and regulations as well as guidelines established by relevant organizations, and conducts internal screening from the viewpoint of the 3Rs principle of animal experimentation, i.e., Replacement (utilization of alternative methods of experimentation that do not involve the use of animals), Reduction (reduction of the number of animals used) and Refinement (alleviation of pain and suffering caused to animals).